
 |
PROTOCOL FOR MANUAL TISSUE MICRODISSECTION
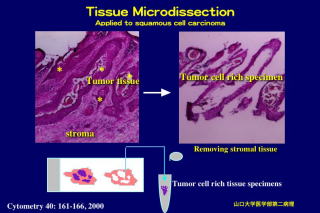
This method is applicable to various kinds of cancer tissues to reduce
stromal tissue components from cancer specimens. Investigators should also
expect to invest time initially by practicing on 10 to 20 cases to begin
to feel comfortable with the technique. As they become skilled in the microdissectiion
technology, they obtain tissue fragments rich in cancer cells in a short
time.
I.
Preparation of frozen tissue sections
1. Set the machine (cryo-stat) for -20
(It takes at
least a few hours to get the optimal temperature. Fortunately, the machine is
usually maintained at -20C in this laboratory.)
2. Squeeze one drop of OCT compound (Tissue Tek) in bottom of mold, then put
@@the tissue specimen in the center of it, and full the mold by OCT compound.
@@@(avoid to trapping air bubbles)
*Embed fresh tissues carefully in OCT in plastic mold, taking care not
to trap air bubbles surrounding the tissue. Freeze tissue by setting mold
on top of liquid nitrogen until 70-80% of the block turns white and then
put block on top of dry ice. The frozen blocks may be stored at minus 80C for long-term@storage.
3. Put mold in machine for 20-30 minutes.
This process can be omitted, when the tissue specimens
frozen by another method are used.
4. Take out the cylinder from machine and placed
one drop of compound
on up of fit for mold
fixation, Convert the mold and put in top of the
cylinder, leave it for 10
minutes in the machine.
5. Remove moldfs
frame (plastic).
6. Adjust the
cylinder for tissue sectioning.
7. Cut the tissue
block with a razor at 5Κm, and put on the slide
nicety.
(A tissue section is prepared for
microscopic observation. When the density of cancer cells is very low, another
tissue specimen should be used.)
8. Then, section the
tissue block at 30Κm.
9. Make totally
12-16 slides with repeat of procedures No. (7 to 8 ).
10. Leave slides in
Ethanol 100% for 5minutes.
11. Stain tissue
sections with HE.
The lower concentration of hematoxylin and eosin may
improve macromolecule recovery.
12. Arrange slides on
the map-wood, and put a cover glass only on 5Κm-
Sections.
We should evaluate the cancer cell content in the
tissue section before beginning microdissection.
II. Tissue microdissection - Picking up the cancer cells
We perform microdissection on a standard inverted microscope (stereomicroscope)
using a 27 gauge needle on a syringe as the microdissecting tool.
Prepare in advance; @1.5ml-eppendorfe
tube
@@@@ A70%-ethanol
@@@@@@@@B1ml-syringe with
27G needle
1. At first, spill a little of Ethanol on the
slide, look for cancer cell
nest, and take out that by
needle of syringe, put it in the eppendorf
tube (tube with 1/2 volume
alcohol).
While viewing the tissue
through the microscope, the cell population of interest should be gently
scraped with the needle. (As another way, first stromal tissues are removed
from cancer tissue specimens, and cancer cell populations are left on the
slide.) The dissected cells will become detached from the slide and form small
dark clumps of tissue that can be collected on the needle. Several small tissue
fragments can be procured simultaneously. Collection of an initial fragment on
the tip of the needle will assist in procuring subsequent tissue. The tip of
the needle with the procured tissue fragments should be carefully placed into a
small PCR tube containing ethanol. Gentle shaking of the tube will ensure the
tissue detaches from the tip of the needle.
2. Centrifuge the tube for 5minutes at 14,000rpm.
3. Remove the alcohol only, soak the tissue fragments in PBS, and centrifuge
the @tube for 5 minutes at 14,000rpm.
4. Remove PBS.
5. Go to the DNA extraction step (Dneasy tissue kit, QIAGEN, or SepaGene,
@@@Sankojunyaku Co., Ltd.)
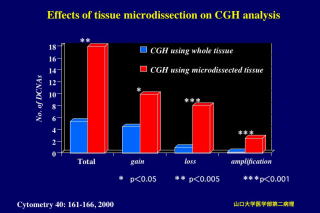
DNA EXTRACTION FROM MICRODISSECTED TISSUE SPECIMENS
DNA extraction is performed with a DNA
extraction kit according to the manufacturerfs instructions. We use two kits
(products of Sanko-junyaku and Quagen) for DNA extraction.
 introduction@ @ introduction@ @ protocols protocols
|
 |
|

