Research about the maturation mechanisms of Type B SDH
Sep 26, 2025: Research_Highlights
Research highlight: The Flavinylation Mechanism of Succinate Dehydrogenase from Gram-positive Bacteria
How the Enzyme’s Own Subunits Drive Its Activation
Authors: Yusuke Shiota and Tomoyuki Kosaka
Key Discoveries
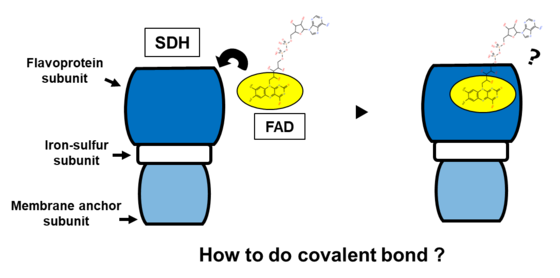
- Elucidated the Activation Mechanism: Revealed that FAD binding in Type B SDH is not spontaneous but requires the assembly of the full enzyme complex—a previously uncharacterized maturation mechanism.
- Achieved Cross-Species Function: Actively expressed the SDH enzyme from the Gram-positive bacterium Corynebacterium glutamicum in E. coli.
- Identified a Critical Bottleneck: Defective maturation of the iron-sulfur subunit is the key bottleneck that prevents other Gram-positive SDHs from functioning in E. coli.
 |
Summary
Succinate dehydrogenase (SDH) is a central enzyme of energy metabolism, and its function critically depends on a process called flavinylation—the covalent attachment of an FAD cofactor. However, how this crucial step occurs in the Type B SDH of Gram-positive bacteria has remained a significant knowledge gap. Our study aimed to address this question by expressing SDH from three different Gram-positive species within an E. coli host.
This approach led to a critical discovery: flavinylation is not a spontaneous event for the FAD-binding subunit alone but is dependent on the assembly of the full enzyme complex. We confirmed this by showing that the presence of the iron-sulfur subunit and fumarate significantly accelerates the reaction in vitro. This work provides new insights into the previously uncovered mechanism of SDH maturation. The implications of our findings extend beyond basic science, offering new strategies for microbial metabolic engineering and paving the way for identifying new physiological pathways.
 |
|---|
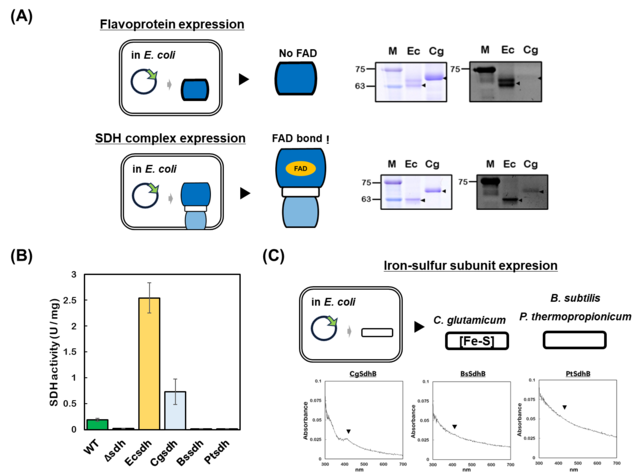
| Fig. 1: Characterization of heterologously expressed succinate dehydrogenase (SDH). (A) Analysis of FAD binding. (B) Comparison of enzymatic activities. (C) Detection of the iron-sulfur cluster in the iron-sulfur subunit. |
Introduction: Addressing a Gap in Understanding SDH Activation
Succinate dehydrogenase (SDH), is an important enzyme complex at the central of metabolism of many organisms. For any SDH to function, the covalent attachment of FAD to its flavoprotein subunit—a process called flavinylation—is the crucial for its succinate oxidizing activity. While the mechanisms for this are well understood for Type A and Type C SDHs, a significant knowledge gap has existed regarding the process in Type B SDH of Gram-positive bacteria.
Our research aimed to address this question. We set out to uncover the flavinylation mechanism of Type B SDH by reconstructing the entire process both in vivo and in vitro, using E. coli as a host system.
Results: Uncovering a Distinct Mechanism
Our investigation yielded several key discoveries. First, we found that the heterologously expressed flavoprotein subunit alone from three different Gram-positive bacteria—Corynebacterium glutamicum, Bacillus subtilis, and Pelotomaculum thermopropionicum—was not flavinylated. The covalent attachment of FAD only occurred when the flavoprotein was co-expressed with its partner subunits, demonstrating that subunit assembly is a prerequisite for its activation (Fig. 1A).
To explore this further, we reconstructed the process in vitro. These experiments revealed that the presence of either the iron-sulfur subunit or the substrate fumarate actively promotes the flavinylation of the Type B SDH.
Interestingly, although FAD binding was successful for all three enzymes, only the SDH from C. glutamicum was functionally active inside E. coli cells (Fig. 1B). By analyzing the purified proteins, we revealed the cause of this failure: the SDHs from B. subtilis and P. thermopropionicum had defects in the insertion of the iron-sulfur clusters into their respective subunits (Fig. 1C). This identified a critical bottleneck in the functional expression of these enzymes in a E. coli host.
Conclusion & Future Outlook
In summary, our work has provided new insights into the previously uncharacterized mechanism for the flavinylationof Type B SDH, demonstrating for the first time that it is a sophisticated process requiring the assembly of the enzyme complex and the presence of its substrate, fumarate.
This fundamental knowledge has significant potential for practical applications in enzyme and metabolic engineering. Furthermore, our study serves as a powerful proof-of-concept, showing that heterologous expression and in vitro reconstruction are highly effective strategies for analyzing the functions of enzymes from microorganisms that are difficult to culture. This approach paves the way for tapping into the vast metabolic potential of the microbial world.
Publication Information
Journal: Bioscience, Biotechnology, and Biochemistry
Title: Insight on flavinylation and functioning factor in Type B succinate dehydrogenase from Gram-positive bacteria
Authors: Yusuke Shiota and Tomoyuki Kosaka
DOI: 10.1093/bbb/zbaf026
Acknowledgements
This research was supported by the Japan Science and Technology Agency (JST) SPRING program (Grant Number JPMJSP2111), the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number 21K05343), and the Institute for Fermentation, Osaka (IFO) (Grant Number LA-2023-018).
Glossary of Terms
- Succinate dehydrogenase (SDH): An enzyme that catalyzes the oxidation of succinate to fumarate. It is the only enzyme that participates in both the TCA cycle and the electron transport chain.
- TCA cycle: A central metabolic pathway that occurs in the mitochondrial matrix of eukaryotes and the cytoplasm of aerobic bacteria.
- Electron transport chain: A system composed of a series of protein complexes and electron carriers located in the inner mitochondrial membrane and the bacterial cell membrane. It is crucial for energy production.
- Prosthetic group: A non-protein, small molecular compound that is tightly bound to an enzyme protein. The specific prosthetic group an enzyme requires is known to be essential for its catalytic activity.
- FAD (Flavin adenine dinucleotide): A type of nucleotide that is an important coenzyme involved in redox reactions. It serves as the prosthetic group for many enzymes, including SDH.
- Gram-positive bacteria: A major group in bacterial classification, distinguished by their cell walls which retain the crystal violet stain in the Gram staining method, causing them to appear purple.
- Corynebacterium glutamicum: A species of Gram-positive bacteria widely used in industry for the production of amino acids, especially glutamic acid.
- Bacillus subtilis: A species of Gram-positive bacteria commonly found in soil and on plants. It is also used for industrial protein production and as a model organism for genetic research.
- Pelotomaculum thermopropionicum: A species of anaerobic, Gram-positive bacteria. It is one of the few species in the world reported to be capable of syntrophic propionate metabolism.